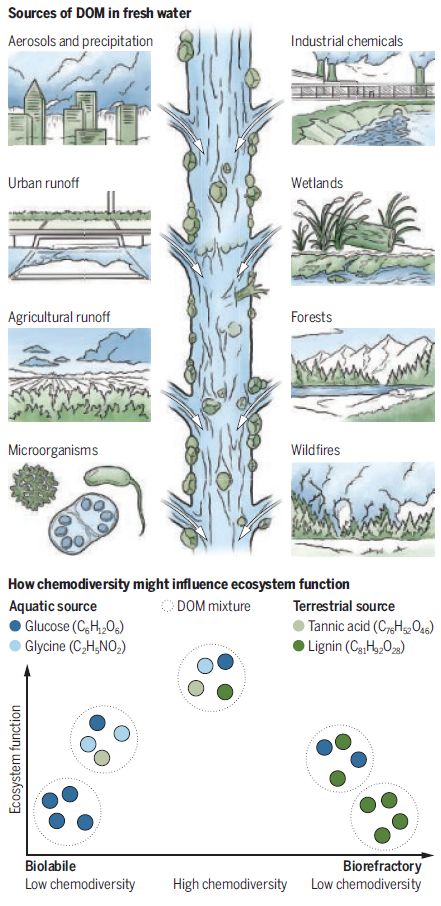
Research increasingly suggests that the health of lakes, rivers and other fresh waters is closely tied to the diversity of dissolved organic matter (DOM). DOM consists of thousands of distinct carbon-based compounds from different plant and animal sources, which make up what scientists call “chemodiversity.”
There are four key ways chemodiversity influences ecosystem functioning:
Nutrient cycling – DOM provides organic carbon and binds nutrients like nitrogen and phosphorus, which can subsidize aquatic food webs or promote harmful algal blooms.
Carbon dynamics – Microbes break down DOM to acquire energy, respiring carbon back to the atmosphere. More recalcitrant DOM can also store carbon in water bodies.
Light penetration – DOM absorbs light which can warm surface waters but limit photosynthesis below.
Toxicity – DOM interacts with contaminants like mercury to affect their accumulation in food webs.
However, traditional measures of DOM bulk properties can mask the diverse roles of distinct compounds. High-resolution mass spectrometry now enables us to assess this “chemodiversity” by resolving molecular composition. This reveals key compound characteristics relating to toxicity, bioavailability and reactivity.
Monitoring chemodiversity could thus transform our ability to diagnose ecosystem health and target interventions. For example, mass spectrometry revealed agricultural runoff, not wastewater, was the chief source of organic phosphorus pollution in the lakes. Such molecular-level insight supports more integrated, catchment-scale solutions.
As human impacts and climate change threaten water quality worldwide, understanding DOM dynamics and leveraging chemodiversity data will be key to protecting ecosystem integrity. I urge member states to prioritize research and monitoring in this critical area.
Bapon (SHM) Fakhruddin, PhD
Water and Climate Leader| Strategic Investment Partnerships and Co-Investments| Professor| EW4ALL| Board Member| Chair- CODATA TG| Award Winner (SDG 2021, EWS 2025)